人工智能辅助技术在胃癌新辅助化疗患者HER2表达评估中的价值
时间:2023-09-23 11:10:16 热度:37.1℃ 作者:网络
[摘要] 背景与目的:胃癌存在较高的肿瘤异质性,部分胃癌患者新辅助化疗(neoadjuvant chemotherapy,NAC)前后人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)表达明显差异化。为了进一步研究HER2表达的异质性,并减少人为评估的视觉误差,本研究采用人工智能(artificial intelligence,AI)辅助显微镜对胃癌患者NAC前后HER2表达进行评估,评价AI的实用性及可行性,并研究NAC对胃癌患者HER2表达的影响,为后续治疗提供参考。方法:收集来自以河北医科大学第四医院为主的多中心、前瞻性、随机对照Ⅲ期临床试验(NCT01516944)中接受NAC的397例胃癌患者临床资料,NAC前胃镜咬检及NAC后手术标本HER2分别采用传统视觉评估及AI辅助显微镜评估,并设定3名高年资病理科医师综合评估结果为金标准。分析两种评估结果与金标准的一致性,探讨影响NAC前后HER2表达变化的临床病理学特征及表达改变对患者预后的影响。结果:相较于视觉评估,AI评估结果与金标准的一致性更佳(0.766 vs 0.853,0.773 vs 0.876)。通过AI评估,97例(24.43%)患者的HER2在NAC后出现表达下调,而27例(6.80%)患者出现HER2表达上调。单因素分析显示HER2表达改变与淋巴结转移(ypN)(P=0.019)、肿瘤退缩分级(P=0.003)明显相关。肿瘤退缩不佳是影响HER2表达上调的独立危险因素(P=0.032)。预后方面,病理学完全缓解(pathologic complete response,pCR)患者5年总生存(overall survival,OS)率、无病生存(disease-free survival,DFS)率均明显优于非pCR患者(92.9% vs 42.5%,P=0.002;92.9% vs 36.0%,P=0.001)。而在非pCR患者中,NAC后HER2表达下调者5年OS率和DFS率均优于上调者(56.7% vs 30.5%,P<0.001;56.1% vs 23.0%,P<0.001);且在非pCR的HER2 2+/3+ 患者中,NAC后HER2表达下调者5年OS率和DFS率均优于HER2表达无改变者(56.7% vs 33.5%,P=0.003;56.1% vs 32.0%,P=0.002)。结论:AI技术应用于胃癌NAC患者HER2评估能减少人为视觉测量误差,可以成为高效、精准评估HER2表达的有力工具。NAC后pCR及HER2表达下调的胃癌患者具有更好的远期生存。若患者HER2表达上调可能提示预后不佳,临床上更应关注此类患者出现复发转移情况,并及时调整治疗方案。
[关键词] 胃癌;新辅助化疗;人表皮生长因子受体2;人工智能
[Abstract] Background and purpose: Tumor heterogeneity exists in gastric cancer. The expression of human epidermal growth factor receptor 2 (HER2) is significantly different in some gastric cancer patients before and after neoadjuvant chemotherapy (NAC). In order to further study the heterogeneity of HER2 expression and reduce the visual error of human interpretation, we used phase Ⅲ artificial intelligence (AI) to assess the HER2 status of gastric cancer patients pre-NAC and post-NAC to evaluate the practicability and feasibility of AI, and studied the effect of NAC on HER2 expression in gastric cancer patients, so as to provide reference for subsequent treatment. Methods: Clinical data of 397 gastric cancer patients receiving NAC were collected from a multicenter, prospective, randomized controlled phase Ⅲ clinical trial (NCT01516944) mainly based on on the Fourth Hospital of Hebei Medical University. HER2 expression in samples of pre-NAC endoscopic biopsies and post-NAC surgical specimens was first assessed visually by 2 pathologists using optical microscope followed by AI-assisted microscope respectively. The comprehensive results of three senior pathologists were set as the gold standard. The consistency between the two assessment results and the gold standard was analyzed, and the clinicopathological features affecting the expression changes of HER2 pre- and post-NAC and the effect of HER2 change on prognosis were also explored. Results: Compared with visual assessment, the consistency between AI assessment results and gold standard was better (0.766 vs 0.853, 0.773 vs 0.876). HER2 expression assessed by AI was down-regulated in 97 patients (24.43%) post-NAC and up-regulated in 27 patients (6.80%). Change of HER2 expression was significantly correlated with lymph node metastasis (ypN, P=0.019) and tumor regression grade (P=0.003). Poor tumor regression was an independent risk factor for the upregulation of HER2 (P=0.032). The 5-year overall survival (OS) rate and disease-free survival (DFS) rate of pathologic complete response (pCR) patients were significantly better compared with non-pCR patients (92.9% vs 42.5%, P=0.002; 92.9% vs 36.0%, P=0.001). In non-pCR patients, the 5-year OS and DFS rates of HER2 down-regulated patients post-NAC were better compared with HER2 up-regulated patients(56.7% vs 30.5%, P<0.001; 56.1% vs 23.0%, P<0.001). And for those HER2 2+/3+ non-pCR patients, the 5-year OS and DFS rates of HER2 down-regulated patients post-NAC were better compared with HER2 unchanged patients (56.7% vs 33.5%, P=0.003; 56.1% vs 32.0%, P=0.002). Conclusion: The application of AI technology in HER2 assessment of NAC patients with gastric cancer can reduce visual measurement error, and AI could be a powerful tool to assess HER2 expression efficiently and accurately. Gastric cancer patients with pCR and down-regulated HER2 post-NAC are more likely to achieve better long-term survival. If upregulation of HER2 status may suggest poor prognosis, attention should be paid closely to the recurrence and metastasis of such patients, and the treatment should be adjusted in time.
[Key words] Gastric cancer; Neoadjuvant chemotherapy; Human epidermal growth factor receptor 2; Artificial intelligence
胃癌是常见的消化道恶性肿瘤之一, GLOBOCAN数据库发布的最新数据显示,2020年新发胃癌数为109万,其中,中国新增胃癌患者约48万(43.9%)[1]。中国地域辽阔,很难实现广泛的胃镜筛查,因而胃癌的早诊率较低,导致就诊时多处于进展期甚至晚期,5年总生存(overall survival,OS)率不及50%[2]。有研究[3]发现抗人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)扩增可能是胃癌患者预后不良的危险因素。ToGA试验证实了抗HER2治疗可改善胃癌或食管胃结合部癌患者的预后[4],临床上逐渐重视其在胃癌诊断及治疗中的地位。
由于肿瘤的多样性和复杂性,使得人们发现HER2在治疗过程中存在明显的异质性表达,这也解释了部分患者并未持续从抗HER2治疗中获益。在经免疫组织化学检测的HER2阳性的胃癌患者中,HER2异质表达发生率为69%~75%[5-7]。HER2表达改变的生物学机制尚不清楚,可能是HER2阳性细胞在内外界因素作用下自我保护转变为阴性细胞,或是细胞毒/靶向药物特异性杀死HER2阳性细胞,令阴性细胞得以存活[8],当然也不除外人为视觉误差造成的主观性评估错误。传统的HER2评估依靠病理科医师显微镜下目视观察肿瘤细胞膜染色强度以及完整性,对医师经验要求高,而且本质上是主观的,容易受观察者间和观察者内变异的影响[9]。随着人工智能(artificial intelligence,AI)技术的进步,计算机算法通过提供的数字图像自动/半自动分析,克服了病理科医师主观性并提高了病理学检查的重复性。AI辅助判读具有稳定性好、重复性高、易于获取及效率高等优点,其排除了传统主观上判读误差的影响,更有利于客观准确地把握肿瘤的HER2状态。
本研究将AI辅助显微镜应用于胃癌新辅助化疗(neoadjuvant chemotherapy,NAC)患者HER2评估中,旨在评价其实用性及可行性,并在AI辅助显微镜的帮助下进一步探讨NAC前后HER2表达变化的影响因素及其对预后的影响,为NAC后HER2表达改变患者后续治疗提供参考。
1 资料和方法
1.1 一般资料
收集2011年1月1日—2016年5月1日以河北医科大学第四医院为主的多中心、前瞻性、随机对照Ⅲ期临床试验(NCT01516944)[10]中接受NAC的397例胃癌患者临床资料,其中男性305例,女性92例,年龄31~77岁,平均年龄(57.5±9.5)岁。所有患者均接受术前2个周期、术后6个周期围手术期化疗,采用XELOX或SOX方案化疗。所有患者入组前均未接受任何抗肿瘤治疗,并排除无法耐受手术,或家属拒绝手术以及化疗前后病理学资料不全的病例。本研究经河北医科大学第四医院伦理委员会批准,所有患者均签署知情同意书。
1.2 研究方法
1.2.1 AI辅助显微镜的建立、验证及使用步骤
本研究采用的AI辅助显微镜在传统显微镜的基础上带有一个增强现实显示器和一个支持AI的计算机单元。AI程序[11]建立过程是采用约500张人工修正的HER2切片典型视野作为训练集。通过热图回归模型应用于完全卷积网络来检测肿瘤细胞,采用骨架化算法从分割掩模中提取轮廓图突出显示染色细胞膜,经验阈值分割图像中的染色膜从而获得细胞膜的完整性和强度。膜染色的完整性和检测到的肿瘤细胞的类别用不同的颜色显示加以区分。最后根据染色强度和细胞膜完整性将检测到的肿瘤细胞分为不同的类别(图1)。训练完成后通过找到预测热图的局部最大响应位置来识别所有的肿瘤细胞。
使用时切片放置于AI辅助显微镜上,低倍镜观察整体情况,通过20倍或40倍物镜选择兴趣视野(filed of interest,FOI),点击AI HER2分析软件中激活按钮便可以在目镜中查看实时AI结果,包括检测到的肿瘤细胞各种染色类别百分比以及HER2建议评分。选取5~8个FOI减小测量误差,软件自动计算所选所有视野中各类染色的细胞所占比例以及染色强度,最终建议评分显示于软件界面。观察者参考建议评分,结合全片评估HER2表达情况。为了方便与其他评估方法区别,之后均以AI评估名称代替AI辅助评估。AI辅助显微镜使用流程见图2。
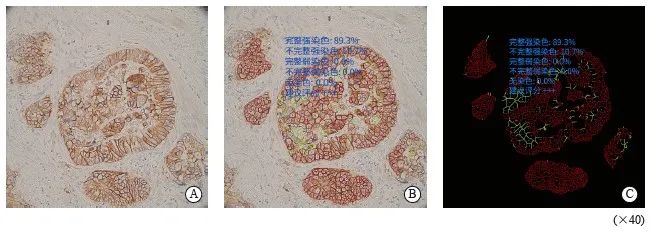
图1 人工智能辅助显微镜评估
Fig. 1 AI-assisted microscope assessment
A: Selected the area; B: Completed convolution network located tumor cells, delineated the stained membranes in different color; C: Shielded the signal of non-tumor cells and highlighted the stained cell membrane. The results showed that the complete and incomplete strong staining rates were 89.3% and 91.7%, the complete and incomplete weak staining rates were 0.0% and 0.0%, the no staining rate was 0.0%, suggested rating +++.
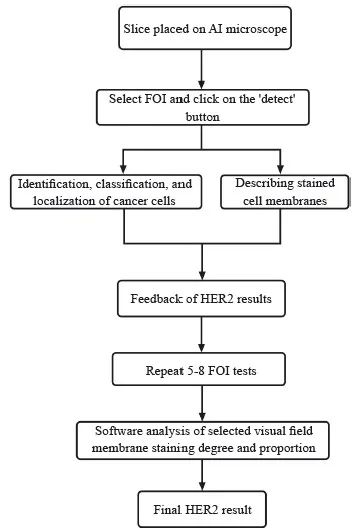
图2 AI辅助显微镜使用流程
Fig. 2 The flow charts of AI-assisted microscope
1.2.2 研究分组
NAC前胃镜咬检以及NAC后手术标本HER2先由2名病理科医师使用普通光学显微镜进行视觉评估,经过2周洗脱期后使用AI辅助显微镜评估。金标准定义为本研究的397个样本先由计算机自动识别细胞的染色和细胞膜完整性,而后由3位高年资病理科医师重新阅片订正未能识别或过度识别的细胞染色和细胞膜完整的个数,按照判读标准重新判读的结果为金标准。HER2表达上调定义为NAC前咬检评分为0或+,化疗后手术标本为++或+++;表达下调定义为NAC前咬检评分为2+/3+化疗后手术标本评分为0或+,或NAC后病理学完全缓解(pathologic complete response,pCR)。本研究中胃癌HER2免疫组化染色流程、评分标准参照美国临床肿瘤学会(American Society of Clinical Oncology, ASCO)及《胃癌HER2检测指南(2016版)》标准进行[12-13]。
1.3 监测及随访
使用增强计算机体层成像(computed tomography,CT)、MRI、胃镜、肿瘤标志物、病理学检查、正电子发射体层成像(positron emission tomography,PET)/CT等多种方式监测患者转移复发情况。随访日期截至2022年1月1日,通过电话、短信、门诊等方式进行跟踪。
1.4 统计学处理
计量资料应用x±s表示,计数资料采用χ2或Fisher确切概率检验。二元logistic回归分析独立危险因素。采用Kappa检验进行一致性分析,其中κ≤0.4、0.4<κ≤0.6、0.6<κ≤0.8、κ>0.8分别表明一致性较差、中度一致性、较高度一致性、一致性极好。生存曲线采用Kaplan-Meier法绘制,单因素分析采用COX回归分析法,将单因素分析中差异有统计学意义(P<0.05)的变量纳入多因素分析。多因素分析采用Cox比例风险回归和风险比(hazard ratio,HR),并计算95% CI,检验水准为α=0.05。OS指从疾病诊断至死亡或随访截止时间,无疾病生存(disease-free survival,DFS)定义为从手术切除到局部复发的时间。采用 SPSS 26.0进行统计学分析,采用GraphPad Prism 8.0.2进行绘图。P <0.05为差异有统计学意义。
2 结 果
2.1 视觉、AI、金标准三种方式评估的HER2表达情况
397例患者视觉、AI、金标准三种评估方式的HER2表达情况见图3。其中14例术后病理学检查为pCR,因此无法检测NAC后手术标本的HER2表达。三种评估方式的NAC前后HER2表达分布差异无统计学意义(χ2=10.737,P=0.097;χ2=11.569,P=0.072)。
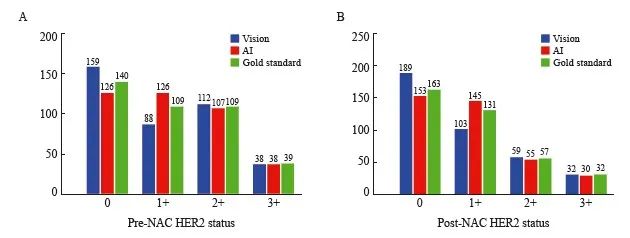
图3 NAC前后视觉、AI、金标准评估的HER2表达
Fig. 3 The pre- and post-NAC HER2 status assessed by vision, AI and gold standard
A: Pre-NAC (P=0.097); B: Post-NAC (P=0.072).
2.2 视觉、AI评估的HER2表达情况与金标准一致性分析
将NAC前后视觉、AI评估的HER2表达情况分别与对应的金标准行一致性分析(图4),在NAC前咬检标本中,视觉、AI评估的HER2与金标准一致率分别为83.38%(331/397)、89.42%(355/397),AI与金标准的一致性优于视觉与金标准(κ=0.853>0.766)。NAC后手术标本中,视觉、AI评估的HER2与金标准一致率分别为84.87%(325/383)、91.64%(351/383),AI与金标准的一致性优于视觉与金标准(κ=0.876>0.773,图4)。临床病理学参数比较见表1。
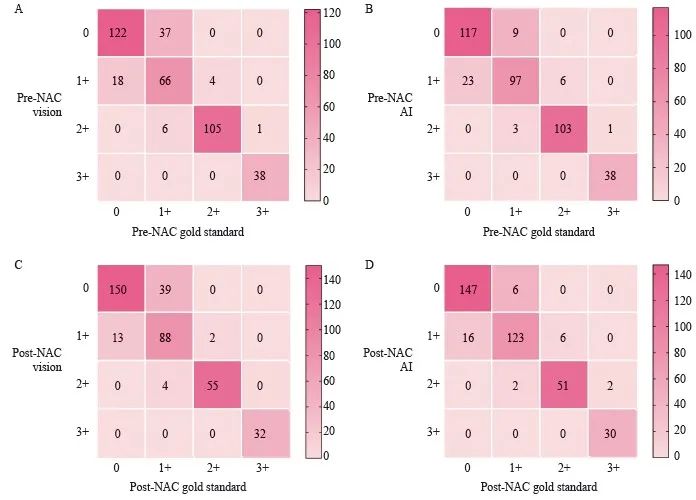
图4 视觉评估、AI评估与金标准评估的一致性比较
Fig. 4 Comparison of consistency between visual assessment, AI-assisted assessment and gold standard assessment
A: Consistency of pre-NAC HER2 between visual assessment with gold standard (κ=0.766); B: Consistency of pre-NAC HER2 between AI assessment with gold standard (κ=0.853); C: Consistency of post-NAC HER2 between visual assessment with gold standard (κ=0.773); D: Consistency of post-NAC HER2 between AI assessment with gold standard (κ=0.876).
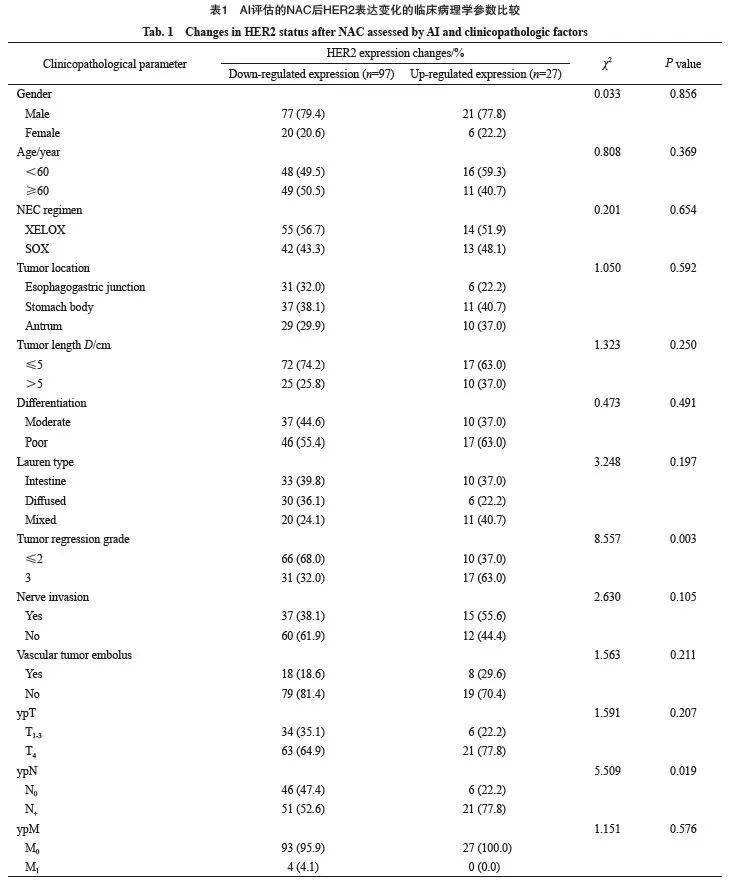
2.3 影响AI评估的NAC前后HER2表达变化的危险因素
397例患者中83例NAC后HER2表达由++/+++ 转变为0/+,14例NAC后达到pCR,表达率下调24.43%;27例由0/+转变为++/+++,表达率上调6.80%。两组间性别、年龄、NAC方案、肿瘤部位、肿瘤大小、分化程度、Lauren分型、神经受侵、脉管瘤栓、ypT分期、ypM分期差异无统计学意义(P>0.05),HER2表达变化与ypN分期(χ2=5.509,P=0.019)、肿瘤退缩分级(χ2=8.557,P=0.003)明显相关(表1)。将ypN分期及肿瘤退缩分级纳入多因素分析,多因素logistic回归分析结果显示,肿瘤退缩不佳是影响HER2表达上调的独立危险因素(P=0.032,OR=2.806,95%CI:1.090~7.222,表2)。
2.4 NAC前后AI评估的HER2表达变化对预后的影响
比较NAC后pCR、非pCR患者生存时间,结果显示pCR患者5年OS、DFS优于非pCR患者(92.9% vs 42.5%,P=0.002,HR=10.98,95% CI:5.88~20.51;92.9% vs 36.0%,P=0.001,HR=13.08,95% CI:7.37~23.21,图5)。
383例非pCR胃癌患者中,有110例患者出现了HER2表达的上调或者下调。结果显示, NAC后HER2表达下调者5年OS、DFS优于上调者(56.7% vs 30.5%,P<0.001,HR=2.81,95% CI:1.31~6.03;56.1% vs 23.0%,P<0.001,HR=2.82,95% CI:1.37~5.81,图6A、7A)。
141例非pCR的HER2 2+/3+患者中,NAC后HER2表达无改变者中位OS、DFS分别为34.0、 22.0个月,5年OS、DFS分别为33.5%、32.0%,均劣于HER2表达下调(P=0.003,HR=2.11,95% CI:1.24~3.57;P=0.002,HR=2.08,95% CI:1.27~3.45,图6B、7B)。242例非pCR的HER2 0/+患者中,NAC后HER2状态无改变者中位OS、DFS分别为35.0、22.0个月,5年OS率、DFS率分别为41.4%、31.4%,与HER2表达上调者生存时间差异无统计学意义(P >0.05,图6C、图7C)。
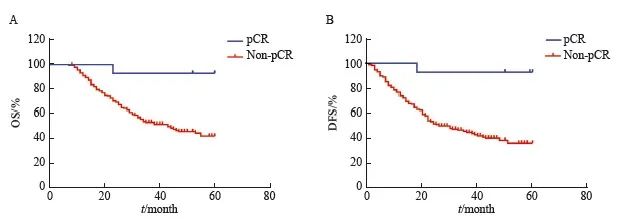
图5 pCR与非pCR患者的OS曲线和DFS曲线
Fig. 5 OS and DFS curves of pCR and non-pCR patients
A: OS (n=397); B: DFS (n=397).
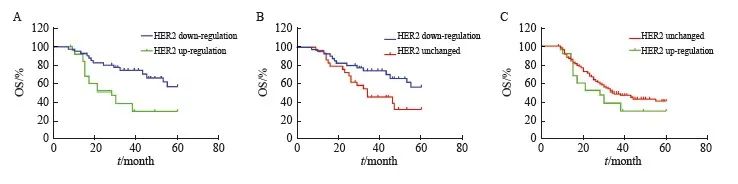
图6 AI评估的NAC后HER2表达变化患者OS曲线
Fig. 6 Impact of the changes in HER2 status after NAC assessed by AI on OS
A: OS (n=110); B: OS (n=141); C: OS (n=242).
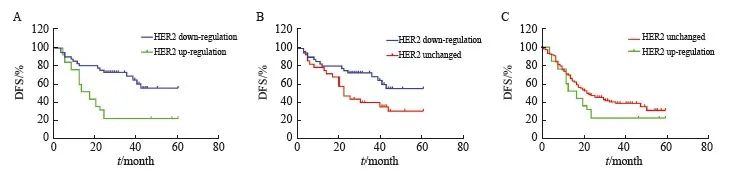
图7 AI评估的NAC后HER2表达变化患者DFS曲线
Fig. 7 Impact of the changes in HER2 status after NAC assessed by AI on DFS
A: DFS (n=110); B: DFS (n=141); C: DFS (n=242).
3 讨 论
过去的十几年中,围手术期化疗、外科手术相结合的胃癌综合治疗体系取得了重大进展, NAC可以有效地缩小肿瘤、降低分期、减轻全身肿瘤负荷,消除潜在的微转移灶,提高手术R0切除率,改善患者预后。国内关于SOX的NAC方案的RESOLVE研究[14]对三种化疗方案(术后XELOX、术后SOX、围手术期SOX)进行比较,证实围手术期化疗可以改善患者3年DFS。而HER2阳性进展期胃癌生物学行为特殊,恶性程度较高,容易复发转移,对常规的细胞毒药物不敏感[15]。多项临床试验[16-17]结果显示,可通过曲妥珠单抗联合两药化疗的治疗模式来使患者获益,且不良反应发生率低。虽然这种治疗模式仍集中于HER2阳性晚期胃癌的一线治疗中,但在新辅助治疗中的应用也在陆续开展。然而传统的胃癌HER2评估仍存在很多问题,比如视觉评估带来的主观误差以及胃癌比乳腺癌更明显的异质性。随着HER2检测规范化、普及化,有研究[18]发现近1/3的胃癌患者NAC后HER2状态较化疗前会发生改变,对于此类患者,重复检测HER2状态会给患者带来经济负担,后续的治疗方案也往往难以决策。随着AI技术的推广,AI技术逐渐运用在病理学诊断上。Yue等[19]为提高HER2评估的准确性及可靠性,将AI技术与传统显微镜结合起来获得评估乳腺癌HER2状态,包含33名病理科医师的三轮环状测试验证了AI的高精度及高重复性。Tuominen等[20]开发了免疫膜程序用于免疫组织化学检测评估HER2的状态,该程序使用颜色去卷积进行染色分离,并使用定制的算法进行细胞膜分割,结合膜染色的强度和完整性生成定量评分,验证集结果显示与病理科医师的评估结果有很好的一致性(kw=0.80),与荧光原位杂交(fluorescence in situ hybridization,FISH)实验及免疫组织化学检测总体不一致率仅为3.5%。该程序还被进一步用于检测HER2 2+病例,结果表明相较于病理科医师初次判读及研究者重复判读,免疫膜程序报告的可疑阳性率明显减少,降低了FISH检测的需求及病理学检查的成本[21]。本研究使用的AI辅助显微镜基于大量数据训练的深度学习,不会产生视觉疲劳,对于存在微弱染色差异的肿瘤细胞有着良好的灵敏度。本研究发现视觉评估、AI评估的HER2与金标准一致率分别为83.38%(331/397)、89.42%(355/397)。AI能够减少视觉评估带来的测量误差,其结果更接近金标准,AI评估较视觉评估的优点在本研究中也得到验证。
NAC前后HER2表达常存在不同程度的变化。Hedner等[22]对166例接受新辅助治疗的食管及胃癌表皮生长因子受体家族进行分析,发现治疗后有5.9%的患者出现HER2状态转变,其中阴转阳1例,阳转阴5例,可能受到病例数限制,并未发现HER2表达改变与肿瘤退缩分级之间的关系。另一项回顾性研究[23]观察到NAC后HER2表达阴转及阳转分别为4例,转变率为1.83%。本研究结果显示,NAC前后HER2存在一定的表达改变,397例患者中83例NAC后HER2表达由2+/3+转变为0/1+,14例NAC后达到pCR,表达下调率为24.43%;27例由0/1+转变为2+/3+,表达上调率为6.80%。进一步比较NAC后HER2表达上调、下调两组间临床病理学特征,结果显示化疗后肿瘤退缩明显,即对化疗敏感的患者HER2表达下调率相应增加。这可能是因为细胞毒性化疗可以提高肿瘤细胞由HER2阳性到阴性的转变几率[24],与HER2阴性胃癌细胞相比,HER2阳性胃癌细胞已被证明具有更高的化疗敏感性[8]。而Pusztai等[25]的研究结果显示,除上述化疗相关因素外,肿瘤内肿瘤细胞之间的异质性同样不容忽视。肿瘤异质性是指同一种肿瘤在不同个体,或同一个患者罹患的不同部位肿瘤从基因型到表型上存在的差异,这导致肿瘤在演变、进展过程中表现出多样性、复杂性。本团队既往研究中发现,对肿瘤病灶进行多点穿刺取材,可以全面评估HER2的状态,减少空间异质性的影响。胃癌患者NAC后HER2表达改变的生物学机制需要进一步的研究阐明。
胃癌NAC前后HER2表达改变与预后关系的相关研究并不多,现有的相似报道大多为回顾性研究。一项大型的回顾性研究[26]纳入了2011年—2018年384例HER2阳性的新辅助靶向联合化疗乳腺癌患者,研究证实综合治疗后HER2转阴及无改变两组间OS、无复发生存(relapse-free survival,RFS)和局部无复发生存(locoregional relapse-free survival,LRFS)情况相似。Seo等[27]研究证实HER2阳性进展期胃癌患者经新辅助靶向治疗联合化疗后,阳性丧失与客观缓解率(objective response rate,ORR)、无进展生存(progression-free survival,PFS)率及OS无关(P>0.05),该研究是一个纳入48例患者的小样本回顾性研究,且中位随访时间仅1年余。本研究结果显示,NAC后HER2表达下调患者5年OS、DFS较表达上调患者延长,且在HER2表达各亚组中,转低表达及转阴者预后优于转阳者。
我国正处于飞速发展时期,现阶段的医疗卫生资源及传统的医学治疗模式难以满足日益增长的卫生服务需求[28],需要新兴技术推动精准医疗、精准诊断发展。AI评估在一定程度上解决了胃癌NAC患者HER2评估过程中人为因素导致的重复性差、观察者内及观察者间误差等系列问题,可提高检测准确性。本研究也存在局限之处,本研究并未对HER2为2+的患者行FISH检测,而是将其与HER2为3+患者归为阳性组,结果可能存在偏差。
综上所述,AI辅助判读应用于胃癌NAC患者HER2评估中稳定、准确率高,可以减少测量误差。NAC后部分患者会出现HER2表达改变,这种变化与患者对化疗的敏感程度相关。NAC后HER2改变与预后相关,表达下调者更有可能从NAC中获益。同时临床医师应持续关注HER2表达上调人群,对其后续治疗方案应力求个体化、精准化。
利益冲突声明:所有作者均声明不存在利益冲突。
[参考文献]
[1] International Agency for Research on Cancer World Health Organization IARC. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012[J]. Globocan, 2012, 2012: 3-6.
[2] STRONG V E, WU A W, SELBY L V, et al. Differences in gastric cancer survival between the US and China[J]. J Surg Oncol, 2015, 112(1): 31-37.
[3] 赵 群, 李 勇, 乔 喜, 等. 胃癌组织中HER2蛋白的过表达与临床病理的相关分析[J]. 肿瘤学杂志, 2015, 21(5): 360-364.
ZHAO Q, LI Y, QIAO X, et al. Correlation between overexpression of HER2 protein and clinicopathology in gastric cancer[J]. J Chin Oncol, 2015, 21(5): 360-364.
[4] BANG Y J, VAN CUTSEM E, FEYEREISLOVA A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial[J]. Lancet, 2010, 376(9742): 687-697.
[5] K U R O K A W A Y , MA T S U U R A N , K I M U R A Y , e t a l . Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer[J]. Gastric Cancer, 2015, 18(4): 691-697.
[6] YAGI S, WAKATSUKI T, YAMAMOTO N, et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer[J]. Gastric Cancer, 2019, 22(3): 518-525.
[7] MOTOSHIMA S, YONEMOTO K, KAMEI H, et al. Prognostic implications of HER2 heterogeneity in gastric cancer[J]. Oncotarget, 2018, 9(10): 9262-9272.
[8] LORDICK F, KANG Y K, CHUNG H C, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial[J]. Lancet Oncol, 2013, 14(6): 490-499.
[9] QAISER T, RAJPOOT N M. Learning where to see: a novel attention model for automated immunohistochemical scoring[J]. IEEE Trans Med Imaging, 2019, 38(11): 2620-2631.
[10] ZHAO Q, LIAN C H, HUO Z B, et al. The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: a multicenter randomized clinical trial[J]. Cancer Med, 2020, 9(16): 5731-5745.
[11] ZHANG J, TIAN K, DONG P, et al. Microscope based HER2 scoring system[EB/OL]. 2020: arXiv: 2009.06816. https:// arxiv.org/abs/2009.06816[2022-09-01].
[12] 《胃癌HER2检测指南(2016版)》专家组. 胃癌HER2检测指南(2016版)[J]. 中华病理学杂志, 2016, 45(8): 528-532.
HER2 Detection Guidelines for Gastric Cancer (2016 edition) Expert Group. HER2 detection guidelines for gastric cancer (2016 edition)[J]. Chin J Pathol, 2016, 45(8): 528-532.
[13] BARTLEY A N, WASHINGTON M K, COLASACCO C, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology[J]. J Clin Oncol, 2017, 35(4): 446-464.
[14] JI J, SHEN L, LI Z, et al. Perioperative chemotherapy of oxaliplatin combined with S-1 (SOX) versus postoperative chemotherapy of SOX or oxaliplatin with capecitabine (XELOX) in locally advanced gastric adenocarcinoma with D2 gastrectomy: a randomized phase Ⅲ trial (RESOLVE trial)[J]. Ann Oncol, 2019, 30: v877.
[15] 殷 科, 曹永晋. 曲妥珠单抗新辅助化疗表皮生长因子受体2阳性乳腺癌的临床疗效及安全性评价[J]. 中国临床药理学杂志, 2015, 31(9): 725-728.
YIN K, CAO Y J. Study on efficacy and safety of trastuzumab chemotherapy for the treatment of human epidermal growth factor receptor-2 positive breast cancer[J]. Chin J Clin Pharmocol, 2015, 31(9): 725-728.
[16] CHUA C, TAN I B, YAMADA Y, et al. Phase Ⅱ study of trastuzumab in combination with S-1 and cisplatin in the firstline treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer[J]. Cancer Chemother Pharmacol, 2015, 76(2): 397-408.
[17] RYU M H, YOO C, KIM J G, et al. Multicenter phase Ⅱ study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer[J]. Eur J Cancer, 2015, 51(4): 482-488.
[18] HE Q F, CHEN J H, ZHOU K, et al. Effect of additional trastuzumab in neoadjuvant and adjuvant treatment for patients with resectable HER2-positive gastric cancer[J]. Ann Surg Oncol, 2021, 28(8): 4413-4422.
[19] YUE M, ZHANG J, WANG X R, et al. Can AI-assisted microscope facilitate breast HER2 interpretation? A multiinstitutional ring study[J]. Virchows Arch, 2021, 479(3): 443-449.
[20] TUOMINEN V J, TOLONEN T T, ISOLA J. ImmunoMembrane: a publicly available web application for digital image analysis of HER2 immunohistochemistry[J]. Histopathology, 2012, 60(5): 758-767.
[21] HELIN H O, TUOMINEN V J, YLINEN O, et al. Free digital image analysis software helps to resolve equivocal scores in HER2 immunohistochemistry[J]. Virchows Arch, 2016, 468(2): 191-198.
[22] HEDNER C, BORG D, NODIN B, et al. Expression and prognostic significance of human epidermal growth factor receptors 1, 2 and 3 in oesophageal and gastric adenocarcinomas preneoadjuvant and postneoadjuvant treatment[J]. J Clin Pathol, 2018, 71(5): 451-462.
[23] WATSON S, VALIDIRE P, CERVERA P, et al. Combined HER2 analysis of biopsies and surgical specimens to optimize detection of trastuzumab-eligible patients in eso-gastric adenocarcinoma: a GERCOR study[J]. Ann Oncol, 2013, 24(12): 3035-3039.
[24] JORDAN N V, BARDIA A, WITTNER B S, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells[J]. Nature, 2016, 537(7618): 102-106.
[25] PUSZTAI L, VIALE G, KELLY C M, et al. Estrogen and HER2 receptor discordance between primary breast cancer and metastasis[J]. Oncol, 2010, 15(11): 1164-1168.
[26] WETZEL C L, SUTTON T L, GARDINER S, et al. Loss of HER2-positivity following neoadjuvant targeted therapy for breast cancer is not associated with inferior oncologic outcomes[J]. J Surg Oncol, 2021, 124(8): 1224-1234.
[27] SEO S, RYU M H, PARK Y S, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3)[J]. Gastric Cancer, 2019, 22(3): 527-535.
[28] 于观贞, 魏培莲, 陈 颖, 等. 人工智能在肿瘤病理诊断和评估中的应用与思考[J]. 第二军医大学学报, 2017, 38(11): 1349-1354.
YU G Z, WEI P L, CHEN Y, et al. Artificial intelligence in pathological diagnosis and assessment of human solid tumor: application and thinking[J]. Acad J Second Mil Med Univ, 2017, 38(11): 1349-1354.


