美国在研的“胃癌”立项关注这些热点,这两项基金令人印象深刻;特别是第二项,对于其他癌症研究更有启发(2024)
时间:2024-09-28 14:03:37 热度:37.1℃ 作者:网络
胃癌(Gastric Cancer)是一种起源于胃黏膜上皮细胞的恶性肿瘤,它可以发生在胃的任何部位,并且可能随时间蔓延至胃外的其他器官。早期胃癌往往没有明显症状,这使得早期诊断较为困难。随着病情发展,可能出现上腹部疼痛、消化不良、食欲减退、体重下降、呕吐和贫血等症状。
尽管近年来在胃癌的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
早期诊断:由于缺乏特异性早期症状,胃癌常在晚期被发现,此时治疗效果较差。开发有效的早期筛查和诊断方法是一个重要的研究方向。
-
个体化治疗:胃癌患者的生物学特性差异较大,需要根据肿瘤的基因型和表型来个体化治疗策略。目前,尽管有一些靶向药物和免疫疗法在使用,但如何为每个患者选择最合适的治疗方案仍是一个挑战。
-
治疗耐药性:随着治疗的进行,部分患者可能发展出对化疗或靶向治疗的耐药性,需要新的策略来克服这一问题。
-
全球治疗不均等:胃癌的发病率和死亡率在全球范围内分布不均,低收入国家的患者尤其缺乏有效的治疗资源和方法。改善这些地区的医疗设施和提高医疗人员的专业水平是公共卫生领域的重要任务。
胃癌的研究和治疗需要多学科的合作,包括肿瘤学、胃肠病学、分子生物学和公共卫生等,通过持续的研究和国际合作,可以提高胃癌患者的生存率和生活质量。
我们仅对美国国立卫生研究院(NIH)资助的在研胃癌相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Gastric Cancer”为检索词、在题目中进行检索,美国NIH针对胃癌的在研有24项。
一,谁获得了这些研究?
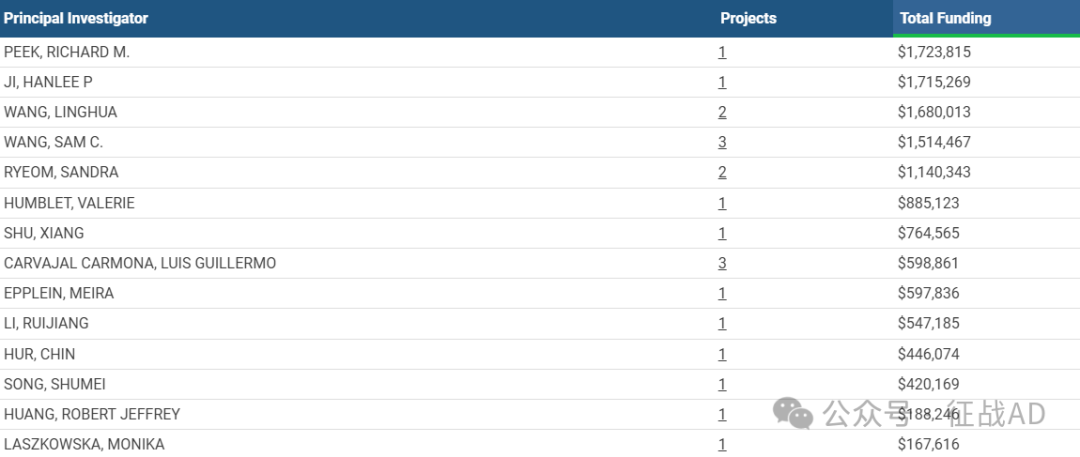
1,在研胃癌基金最多的PI
-
VANDERBILT UNIVERSITY MEDICAL CENTER 的 PEEK, RICHARD M.
-
STANFORD UNIVERSITY 的 JI, HANLEE P
-
UNIVERSITY OF TX MD ANDERSON CAN CTR 的 WANG, LINGHUA
-
UT SOUTHWESTERN MEDICAL CENTER 的 WANG, SAM C.
-
COLUMBIA UNIVERSITY HEALTH SCIENCES 的 RYEOM, SANDRA
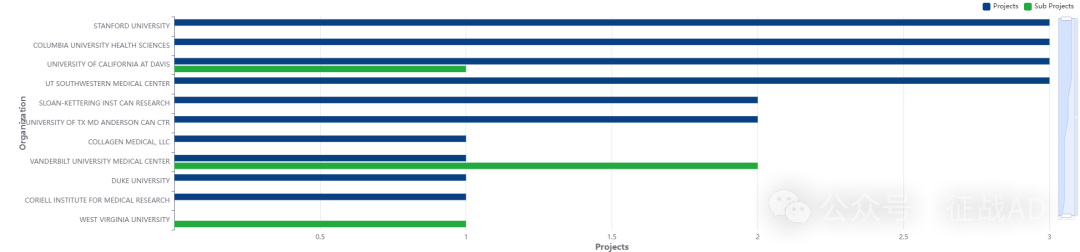
2,胃癌基金最多的研究机构
-
斯坦福大学
-
范德堡大学医学中心
-
德克萨斯大学 MD 安德森癌症中心
-
哥伦比亚大学健康科学系
-
德克萨斯大学西南医学中心等
二,胃癌研究热点是什么?
胃癌研究领域总览(根据关键词)
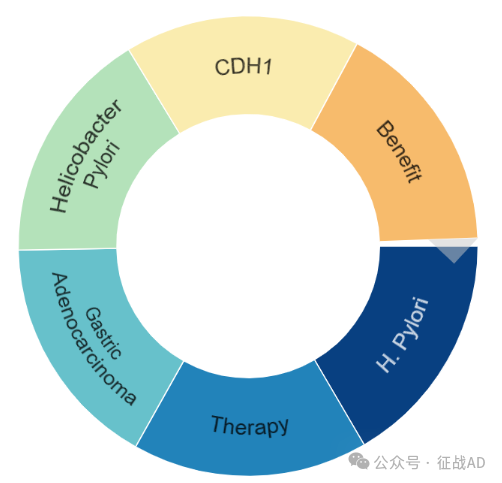
胃癌研究大的方向主要包括治疗(Therapy)、益处(Benefit)、胃腺癌(Gastric Adenocarcinoma)、CDH1、幽门螺杆菌(Helicobacter Pylori)等。
三,借鉴与突破
我们也分享在胃癌领域的几项课题摘要,希望对同仁们有所启发。
A,H. pylori-induced Inflammation and Gastric Cancer
Helicobacter pylori is the strongest risk factor for gastric adenocarcinoma, the fourth leading cause of cancer- related death. One H. pylori determinant that increases gastric cancer risk is the cag type IV secretion system (T4SS) which exports a bacterial oncoprotein, CagA, into host cells. Our entire group has collaboratively shown that cag+ strains selectively activate a gastric stem cell population marked by Lrig1, as well as the EGF receptor, ornithine decarboxylase, and spermine oxidase, host effectors that influence carcinogenesis. Project 2, Core A, and Core B have additionally made the discovery that a pathway contributing to gastric carcinogenesis involves reactive electrophiles and developed a novel intervention strategy using a clinically available electrophile scavenger.Projects 1 and 3 demonstrated with Cores A and B that environmental components of the exposome associated with gastric cancer, such as iron deficiency or a high salt diet, positively select for H. pylori variants linked to increased cancer risk and augment the ability of cag+ strains to induce disease. Finally, all Projects used unbiased approaches (Core B) to identify novel host effectors that increase cancer risk, including bile acids. Our overarching Hypothesis is that differences in infecting strains, host responses, and environmental exposures such as diet affect the risk of developing gastric cancer as a consequence of H. pylori infection.
To address this, our PPG integrates studies of host-pathogen interactions and oncogenic signaling initiated by biomedical researchers who have made a strong commitment to research within the fields of carcinogenesis, immunobiology, gastroenterology, and microbiology, and will generate results that would not be attainable through independent investigation. The Projects below are driven by discrete hypotheses, yet are cohesive in their focus on H. pylori-host interactions that induce cellular responses with carcinogenic potential.
Project 1. Effect of iron deprivation on H. pylori-induced gastric carcinogenesis (PI-Richard Peek)
Project 2. Polyamines and electrophiles in gastric cancer (PI-Keith Wilson)
Project 3. Regulation of H. pylori virulence by dietary factors that impact gastric cancer (PI-Tim Cover)
The efforts of each Project will be further unified by dynamic interactions with specific Core facilities, consisting of Gastric Histopathology Core A, Proteomics and Metabolomics Core B, and Administrative Core C, which includes sophisticated bioinformatics and statistics.
B, Theranostic for gastric cancer
Building upon this preliminary data, in this FastTrack project we propose to establish a theranostic approach to treating GCa by using 64Cu-CM500 PET to identify patients with fibrin-rich gastric tumors and then treating them with fibrin-targeted 90Y-CM600 beta emitting radiotherapy.
In Phase 1 we will evaluate the extent of fibrin deposition in a wide range of human GCa tissue arrays to estimate the prevalence of fibrin in GCa. We will also demonstrate the specificity of our compounds for both detecting tumor fibrin and for targeting fibrin for radiotherapy.
In Phase 2 we will evaluate the kinetics of 64Cu-CM500 PET in tumors in GCa patients and estimate how long the probe remains bound to the tumor. We will also demonstrate the efficacy of 90Y-CM600 radiotherapy in multiple mouse models of GCa and optimize the treatment regimen.
Lastly, to enable clinical translation of this therapeutic, we will synthesize the CM600 precursor under cGMP conditions, validate the 90Y radiolabeling method, and perform IND enabling GLP toxicity, biodistribution and dosimetry studies in rodents.


